So last time i was here, we were discussing the bio-signifance of Carbohydrates.
On tonight’s Biochem broadcast; we will be covering the following top stories:
- Monosaccharides
- Disaccharides
- Polysaccharides
I am your presenter for this evening; my name is Shan Fred.
I was informed earlier today that the famous family “Monosaccharide” is indeed the simplest sugar unit of a Carbohydrates.
To cover this story in depth we shall go over to Stacii Fred, who will present to us from a Biochemist perspective.
The family Monosaccharide can be further classified into two groups: Aldehydes and Ketones; more specifically aldoses and ketoses.
For those who are not doing chemistry, i will explain what a carbonyl group is, since it is necessary to understand the classification of aldose sugars and ketose sugars.
- Carbonyl group: it is a functional group that is recognized by a carbon atom being doubly bonded to an oxygen atom.

We will use the carbonyl group to differentiate between an Adlose sugar and a Ketose sugar:
- Aldose Sugar: the carbonyl group is at the end of the Monosaccharide chain (last member of the family): Example:the most famous daughter GLUCOSE:

Note the position of hydroxyl group (OH) on the 5th Carbon atom; which is the chiral carbon furthest away from the carbonyl group. Glucose has 4 chiral carbons. Based on the report in front me, a Chiral carbon (asymmetric carbon) is defined as a compound which has four different groups bonded to it, therefore the atoms are arranged differently in space thus resulting in optically active isomeric forms called Enantiomers. When the OH group is on the left this is L-designation, therefore L-Glucose and when its on the right it is D-Designation therefore D-glucose.
- Ketose Sugar: the carbonyl group is within the chain structure. i.e. any other carbon that is not at the end. However it is usually at carbon 2. An example would be Fructose the jealous sister:

Notice the position of the carbonyl group(C-2); and also the position of the chiral carbon furthest away from the carbonyl group(C-5).
All the structures above for glucose and fructose are in the Fischer projection form.
Now we are going to focus on the Haworth Projections:
These structures are derived from the cyclization of the Monosaccharides, which is as a result of a reaction between the aldehyde or ketone group and an alcohol group. The structures formed are either:
- Hemiacetal——–> if the reaction is between an aldehyde and alcohol
- Hemiketal———> if the reaction is between a ketone and alcohol.
Since we have been looking at glucose and fructose; They will be the candidates for cyclization.
The Cyclization of Glucose occurs between the aldehyde group on C1 and the OH group on C5. Glucose forms a 6 member ring hence it is referred to as pyranose ring. A new asymmetric carbon is formed at C1 due to the cyclization, thus 2 new stereo-isomers called anomers are formed: α and β.
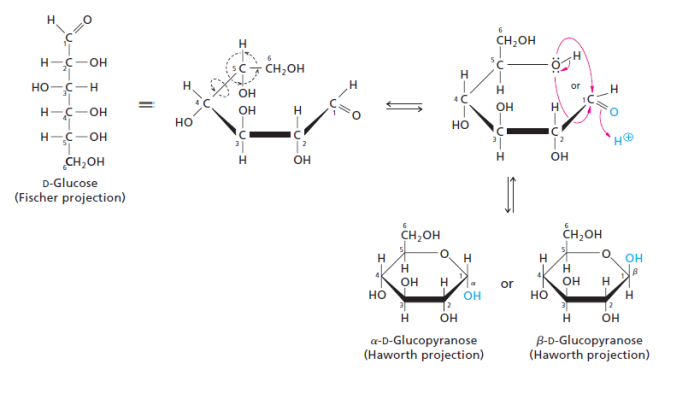
(Moran et al 2012, 232).
The structure is deemed α or β based on the position of the OH group which is attached to the anomeric carbon. If the OH group is above the plane of the ring, it is β; if the OH group is below the plane of the ring it is α.
NB: the anomeric carbon for cyclized glucose is C1
The cyclization of fructose occurs between the ketone group at C2 and the OH group at C5, which produces a 5 member ring called furanose. A new asymmetric carbon is formed at C2 thus resulting in the formation of α and β structures. The same theory applies for the identification of the alpha or beta structure of fructose.
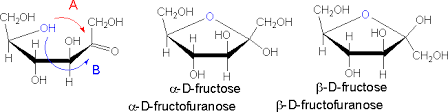
NB: the anomeric carbon for cyclized fructose is at C2
Disaccharides:
They are produced via a condensation reaction, which occurs between two monosaccharides. The OH group of one monosaccharide combines with the Hydrogen group of another, producing a water molecule. The two monosaccharides are now covalently bonded by an oxygen bridge called a Glycosidic Bond.
The 3 major disaccharides we should be aware of are:
- Maltose: formed via a condensation reaction between two α Glucose monosaccharides.

The Glycosidic bond formed: α (1-4) .
As you can see from the diagram, the bond is formed between the OH group on C1 of one Glucose molecule and C4 of the other.
- Lactose: formed via a condensation reaction between β Glucose and β Galactose.

Glycosidic bond formed: β(1-4)
- Sucrose: formed via a condensation reaction between β Fructose and α Glucose.

Glycosidic Bond formed: (α1-2β)
As you can see from the diagram the bond formed is between C1 of Glucose and C2 of Fructose.
Polysaccharides:
These are polymers of monosaccharides. The 3 major structures we should be aware of are ALL polymers of Glucose:
- Starch: contains two types of Glucose polymers: Amylose and Amylopectin;
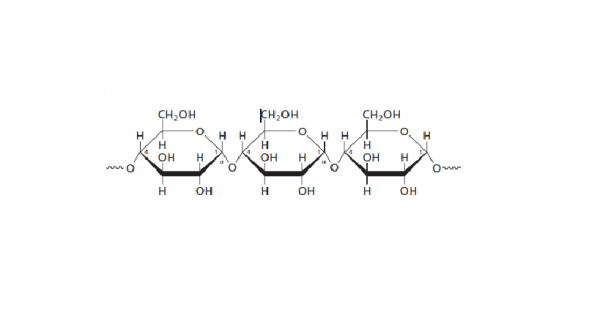
(Moran et al 2012, 241).
Amylose is unbranched, with α(1-4) linkages between Glucose molecules.
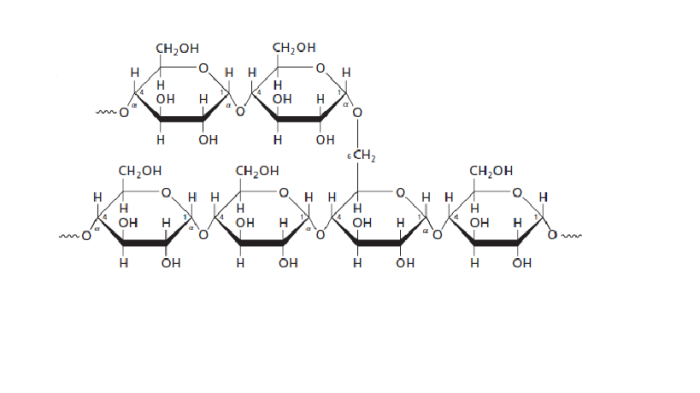
(Moran et al 2012, 242).
Amylopectin is branched with α(1-4) and α(1-6) linkages between the Glucose molecules
- Glycogen: the structure resembles that of Amylopectin, however there are more α(1-6) branches present in glycogen.
 This is a branched structure with α(1-4) and α(1-6) linkages between the Glucose molecules.
This is a branched structure with α(1-4) and α(1-6) linkages between the Glucose molecules.
- Cellulose: is composed of β(1-4) linkages. In this structure every other glucose molecule is flipped over to accommodate the β linkages.

This is an unbranched structure.
As we come to the end of our broadcast I would like to thank our sponsors:
Google Images: all diagrams were obtained from Google Images unless otherwise stated.
Moran, Laurence A, H. Robert Horton, K. Grey Scrimgeour, Marc D. Perry. 2012. Principles of Biochemistry. 5th ed
Cox, Michael, and David L. Nelson. 2008. Lehninger Principles of Biochemistry. 5th ed. New York
That will be all for the night! Hope my presentation was informative. Back to you Shan Fred. Goodnight!
Be the change you want to see in the world!
He who changes one person, changes the world entire!



















